Protect your endoscopes
during transport, storage and sterilization
Reduce costs - Increase protection
Standard products
cascadable variants
- EndoGuard® ESK190-C1-10 (for Storz system / L=190mm)
- EndoGuard® ESK209-C1-10 (for Storz system / L=209mm)
non-cascadable variants
- EndoGuard® ESN190-C1-10 (for Storz system / L=190mm)
- EndoGuard® ESN209-C1-10 (for Storz system / L=209mm)
- EndoGuard® ESN320-C1-10 (for Storz system / L=320mm)
Attractive B2B Conditiones
EndoGuard® in medical practice
Endoscopy procedures are used in countless areas. One of the most demanding applications of endoscopy is in medicine. EndoGuard® has been developed for use in the medical field and reliably protects endoscopes for applications such as those listed below.
Gastroenterology
Duodenoscopy: Endoscopy of the duodenum
Enteroscopy: Endoscopy of the small intestine
Gastroscopy: endoscopy of the stomach
Colonoscopy: endoscopy of the large intestine
Oesophagoscopy: endoscopy of the oesophagus
Proctoscopy: Endoscopy of the anal canal
Rectoscopy: endoscopy of the rectum
Pneumology
Bronchoscopy: endoscopy of the trachea
Urology
Cystoscopy, urethrocystoscopy: endoscopy of the urethra and bladder
Surgery
Laparoscopy: Endoscopy of the abdominal cavity
Gynaecology
Hysteroscopy: Endoscopy of the uterus
Colposcopy: Endoscopy of the vagina and cervix
Orthopaedics
Arthroscopy: endoscopy of the joints
ENT medicine
Laryngoscopy: endoscopy of the larynx
Otoscopy: endoscopy of the auditory canal and eardrum
Pharyngoscopy: endoscopy of the pharynx
Rhinoscopy: Endoscopy of the nose
To ensure that highly sensitive medical endoscopes and their functional units or optics always function perfectly, reliable protection is essential throughout the entire logistics chain, right up to the prepared place of use.
There is a tendency for many of the processes that take place between the operative use of medical endoscopes to be shifted to remote system providers. Therefore, robust transfer packaging adapted to the high-quality goods being transported is gaining in importance.
EndoGuard® endoscope protection sleeves offer handling and cost advantages due to the possibility of carrying out different sterilisation processes "in housing" - i.e. in the stable packaging without removal and the associated risks.
EndoGuard® product information
The original type of EndoGuard® endoscope protective sleeves, which have been in proven use for over 25 years, was developed and manufactured in Berlin.
The shift in use from pure protective tubes for sterilisation purposes to robust packaging for high-quality rigid endoscopes is actively accompanied by constant engineering and flows into new product variants.
EndoGuard® endoscope protective sleeves represent a low-cost alternative to products from established medical device manufacturers in the market segment. (Customer feedback from Post-Market Surveillance 2021)
The EndoGuard® endoscope sheaths are designed to be highly resistant to external influences, provide reliable locking of the transport/sterilisation goods in combination with robust, high-quality materials and technologically and qualitatively demanding manufacturing processes, and offer functional advantages characterised by a high degree of reliability for all intended applications.
The resistance to numerous chemicals, various diluted acids and alkalis as well as aliphatic, aromatic and halogenated hydrocarbons as well as oils and alcohols characterise the specially treated plastic. In addition to its high mechanical strength, rigidity, impact resistance and hardness, the material used also offers high resilience. This enables a repeatable safe use of the locking function and ensures a secure hold - even under extreme operating conditions and logistics requirements.
Technical parameters
- maximum diameter of the optics: 5.00mm (recommended)
- inner diameter: 6,25mm (minimum)
- mechanical locking: bayonet lock (according to compatibility)
- maximum storage temperature: 40°C
- nominal life: 6 months (after first use)
- risk class: class I (RL 93/42/EWG "MDD", replaced by VO (EU) 2017/745 "MDR")
- Material: thermoplastic material
Permissible sterilisation methods
- Autoclaving / steam sterilisation: max. T = 134°C / up to 60 processes
- Formaldehyde gas sterilisation (FO): max. T = 80°C / up to 60 processes
- Ethylene oxide sterilisation (EO/CO2): max. T = 80°C / up to 30 processes
- Plasma sterilisation (H2O2): max. T = 60°C / up to 30 processes
- Gamma ray sterilisation (cobalt-60): max. T = 45°C / up to 5 processes
- Beta radiation sterilisation: max. T = 45°C / up to 5 processes
Only limited suitability for radiation and plasma sterilisation!
Due to the high permeation capacity of EO, sufficient expulsion of the active agent (e.g. by CO2) must be ensured for ethylene oxide sterilisation!
Useful life is reduced due to the higher material stress!
Any use beyond the above conditions is at the user's own risk and is expressly not recommended by the manufacturer / distributor for reasons of material stress!
EndoGuard® White labelling
We produce your own individual version of our proven EndoGuard® endoscope protection sleeves.
For this purpose, we offer you many possibilities to adapt the design to your individual requirements, a labelling corresponding to your CI as well as the option of DropShipping to reduce your logistics costs.
Your individualisation options
any colour code
2 logo fields a' 20,50mm x 6,00mm
logo by abrasion-resistant material enhancement (engraving of a font)
printing (e.g. pad printing independent of position)
Laser engraving (position-independent colour change depending on material and colour)
Part processing
Adaptation of the total length (so far products from 32.5mm - 410mm realised)
Adaptation of inner and outer diameters (front side, up to 35mm depth)
Adaptation of the device mounting / locking (e.g. for use with other docking systems)
- Packing and shipping
Number of units per VPE
Labelling of the packaging units
DropShipping (shipping directly to your customer)
EndoGuard® Instructions for use
For the safe use of EndoGuard® endoscope protection sleeves, within the scope of the following intended purpose, the following instructions must be observed.
- mechanical protection during sterilisation procedures of endoscopes, cystoscopes and urethroscopes
- transport and storage packaging of endoscopes, cystoscopes and urethroscopes
Instructions for use
Cleaning and disinfection
Maintenance
Warning and safety instructions
Disposal
Questions or suggestions about EndoGuard® endoscope protection sleeves
Neue Produktideen suchen Feedback
EndoGuard® Schutzkappen > Cap

Viele zufriedene Kunden nutzen zum Schutz von Okulartrichter und Lichtleiteranschluss von uns angebotene individuelle Schutzkappen. Gern würden wir diese praktischen Schutzkappen in Verbindung mit unseren bewährten Schutzhülsen in das Standardsortiment aufnehmen.
Sehen Sie einen Bedarf für die EndoGuard® Schutzkappen > Cap in Ihrem Anwendungsfall?
EndoGuard® Verbindungselemente > Clip
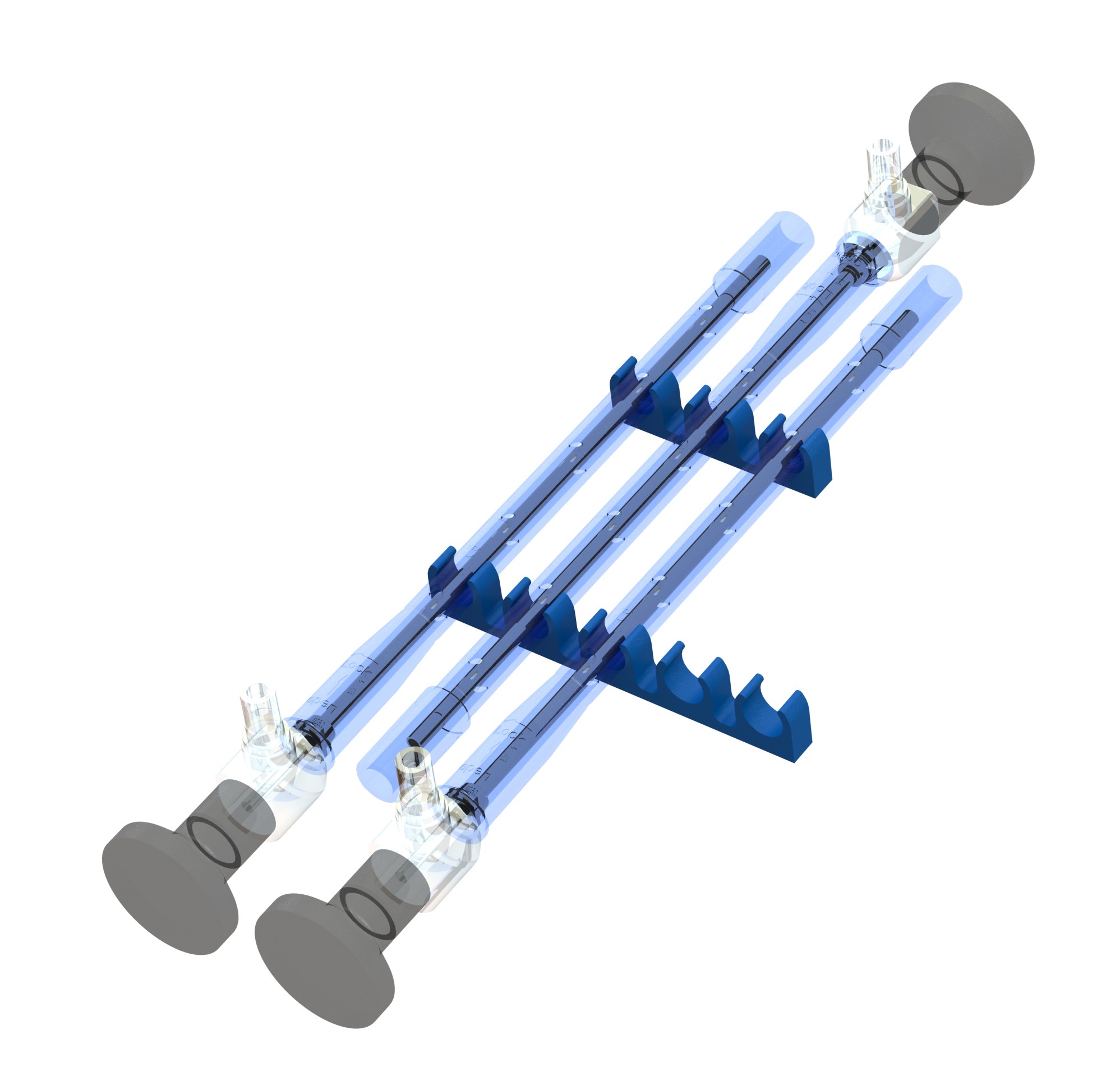
Praktische Anordnung und Vermeidung von gegenseitigen Beschädigungen in Mehrfachverpackungen durch fixierte Abstände der durch EndoGuard® geschützten Endoskope. EndoGuard® Clip Typ A ermöglicht sehr einfache Verpackungsdesigns da unterschiedliche Endoskope mit nur einer Standardverpackungsform sicher und optisch ansprechend umhüllt werden können. Für unsere Kunden bedeutet dies ein hohes Einsparpotential bezüglich der Verpackungskosten.
Sehen Sie einen Bedarf für die EndoGuard® Verbindungselemente / Abstandshalter Clip in Ihrem Anwendungsfall?



